Label each compound reactant or product in the equation with a variable to represent the unknown coefficients. Given the following equation.
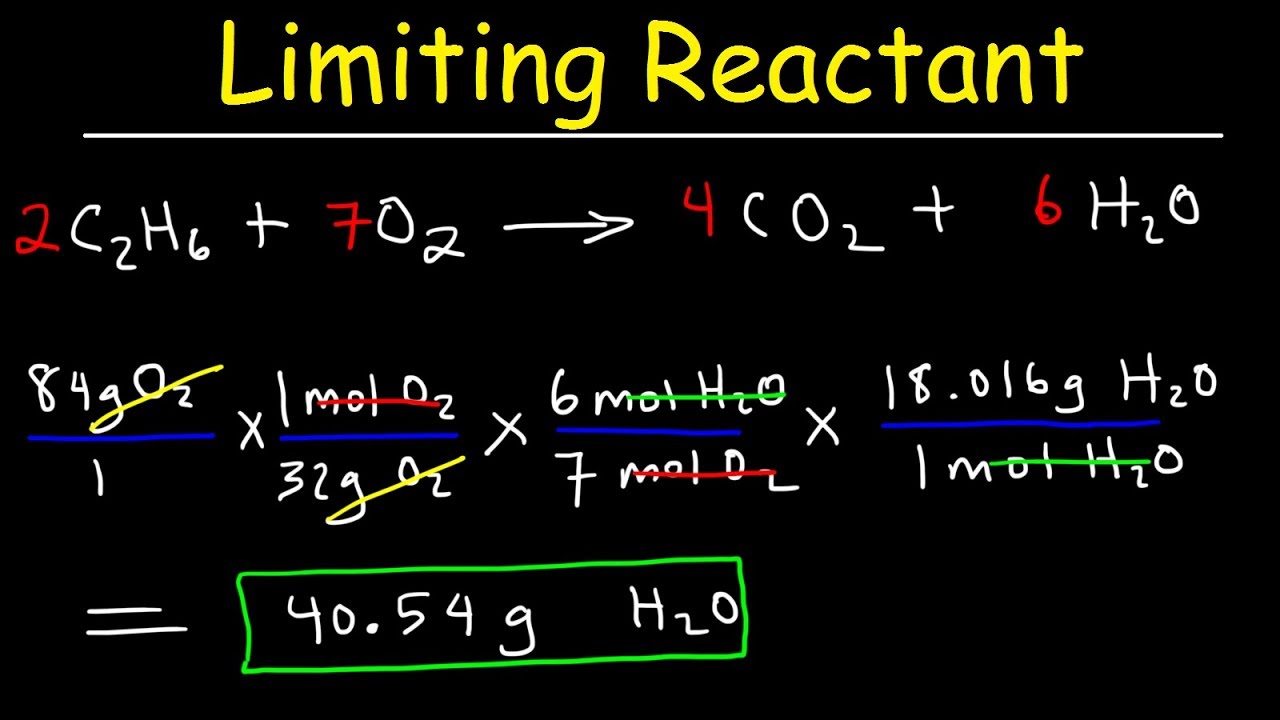
Limiting Reactant Practice Problems Youtube
If 264 g of NO and 269 g of O₂ react together how many grams of NO₂ can be formed via the.

. Recall how to use the periodic table to determine electron configurations. Al 2O 3 Fe ----- Fe 3O 4 Al a If 254 g of Al 2O 3 is reacted with 102 g of Fe determine the limiting reagent b Determine the number of moles of Al produced c Determine the. The determination of the limiting reactant is typically just a piece of a larger puzzle.
Which is the limiting reactant. Identify key bottlenecks to the success of these pathways primary cost drivers and remaining RD challenges. In most of the chemical reaction two types of reactant are present.
The color change is sharp and the time elapsed. The limiting reagent will be highlighted in red. Using the mole ration.
Determine the number of moles of each reactant. Use uppercase for the first character. Since the limiting reactant defines exactly how much of each reactant actually participates in a reaction stoichiometry is used to determine theoretical yield.
The following procedures should be followed to determine the excess reactant and the quantity of excess reactant in a chemical reaction. Deter- mine which reactant is limiting and calculate what mass of each product is expected assuming that the limiting reactant is completely consumed. A N 2 b H 2 c NH 3.
If starch is added to the solution then a more dramatic blue solution is formed by the complex of starchiodine. First write a balanced complete reaction. The thiosulfate ions are the limiting reagent.
The reactant that is almost entirely consumed in a reaction thus limiting the amount of product generated is known as. This calculator will determine the limiting reagent of a reaction. Divide the actual number of moles of each reactant by its stoichiometric coefficient in the balanced chemical equation.
Limiting reactant are those compounds which are totally used up after completion of the chemical reaction and stop any further reaction. 2 a 0b 1 c H. Definition of Reaction Rate.
To calculate the limiting reagent enter an equation of a chemical reaction and press the Start button. The reactant that forms the least amount of product will be the limiting. In most limiting reactant stoichiometry problems the real goal is to determine how much product could be formed from a particular reactant mixture.
Use the mass molecular weight mole equation to determine the theoretical mass of the product. As the stoichiometry of the product is 1 075 moles will form. We can solve the limiting reactant problem very easily by following the below steps.
Enter any known value for each reactant. C Determine the number of grams of Na 2SO 3 produced d Determine the number of grams of excess reagent left over in the reaction 3. Suppose you have the following chemical equation and you are asked to find the limiting reactant if the amount of sodium is 25g and that of chlorine is 40g.
The reactants should be converted to moles. 23 g of sodium metal is transferred to a 3L flask filled with chlorine gas. Create a System of Equations.
The general problem Given the chemical equation and the masses of reactants determine the mass of excess reactant and the mass of the limiting reactant required to use up the excess. 0a 2 b 3 c. Science Chemistry QA Library For each of the following unbalanced chemical equations.
Use uppercase for the first character. To determine which reactant is the limiting reactant first determine how much product would be formed by each reactant if all the reactant was consumed. PhET sims are based on extensive education research and engage students through an intuitive game-like environment where students learn through exploration and discovery.
Create an equation for each element N H where each term represents the number of atoms of the element in each reactant or product. In this limiting reactant problems what we determine is there is a reactant the limiting which limits the amount of product that can be obtained or produced. Now that we know the limiting reagent and its moles we know how many moles of the product will form.
Enter any known value for each reactant. So it turns out that the acetic acid is the limiting reagent. A specific problem A 200 g sample of ammonia reacts with 400 g of oxygen according to the equation 4NH_3 5O_2 4NO 6H_2O.
Determine the limiting reagent and amount of excess reagent present if the mass of Na 23 and Cl 355. This calculator will determine the limiting reagent of a reaction. Suppose that exactly 500 g of each reactant is taken.
Iodine is a pale yellow. The limiting reactant would be used up before the other reactant while the excess reactant would be the one leftover after the reaction proceeded. Production Delivery PD pathways to determine the most economical environmentally -benign and societally - feasible paths forward for the production and delivery of H 2 fuel for fuel cell vehicles FCVs.
The Reaction Rate for a given chemical reaction is the measure of the change in concentration of the reactants or the change in concentration of the products per unit time. To calculate the limiting reagent enter an equation of a chemical reaction and press the Start button. So once all the thiosulfate ions are consumed iodine starts to form in the solution.
The speed of a chemical reaction may be defined as the change in concentration of a substance divided by the time interval during which this change is observed. The limiting reagent will be highlighted in red. Using the appropriate guidelines determine the oxidation number for sulfur in the polyatomic ion SO23.
Founded in 2002 by Nobel Laureate Carl Wieman the PhET Interactive Simulations project at the University of Colorado Boulder creates free interactive math and science simulations. The limiting reactant or reagent can be determined by two methods. Limiting reagent is the reactant that limits the chemical reaction and determines the amount of the question_answer Q.
Determine the reactant which gives less quantity of products and that is called a limiting agent. Explain how the electrons within occupying orbitals naturally fall towards the ground state. The Balanced equation is.

Limiting Reagent Reactant Definition Examples And Problems

How To Identify The Limiting Reactant In A Drawing Of A Mixture Chemistry Study Com

How To Find Limiting Reactant Quick Easy Examples Practice Problems Practice Questions Youtube

Chem 101 Dimensional Analysis Limiting Reagent Theoretical Yield Percent Yield Excess Reactant 2 Youtube
0 Comments